Using femtosecond laser to induce micro/nanostructures on the surface of stainless steel plates and injection needle nozzles
Release Time:
Nov 09,2021
After femtosecond laser treatment, immerse the sample in a 0.01 M stearic acid ethanol solution for 12 hours to reduce surface energy. Generally speaking, metal surfaces are prone to oxidation during femtosecond laser ablation. The COOH group in stearic acid can adsorb onto the natural oxide surface of laser ablated stainless steel, forming a self-assembled monolayer.
After femtosecond laser treatment, immerse the sample in a 0.01 M stearic acid ethanol solution for 12 hours to reduce surface energy. Generally speaking, metal surfaces are prone to oxidation during femtosecond laser ablation. The COOH group in stearic acid can adsorb onto the natural oxide surface of laser ablated stainless steel, forming a self-assembled monolayer.
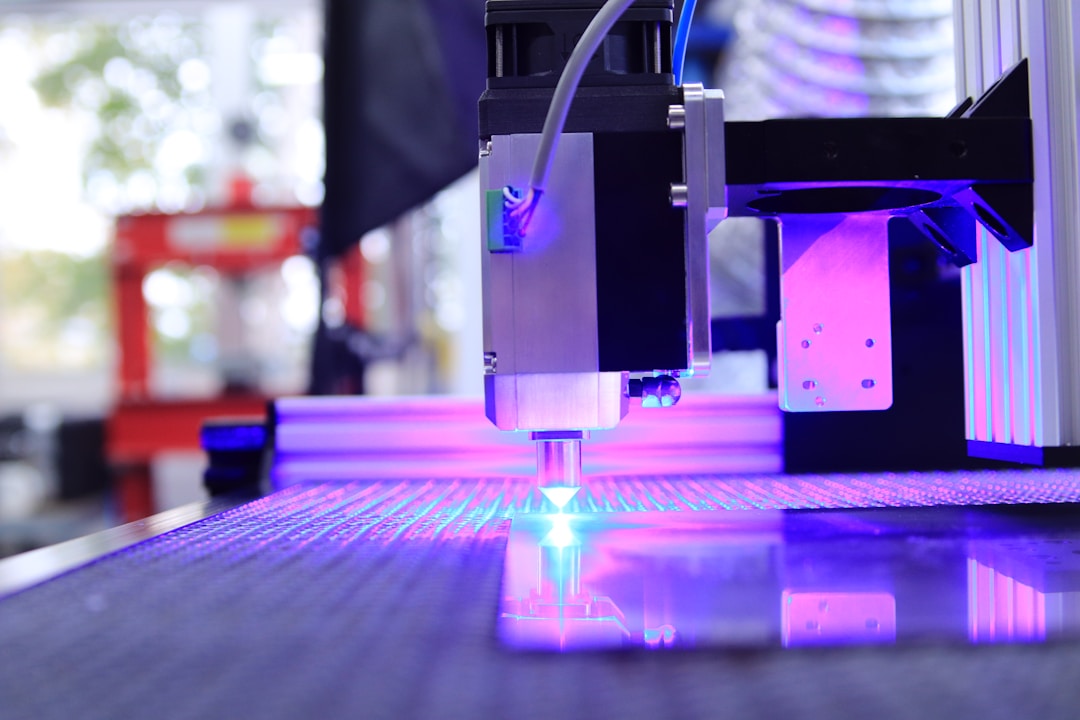
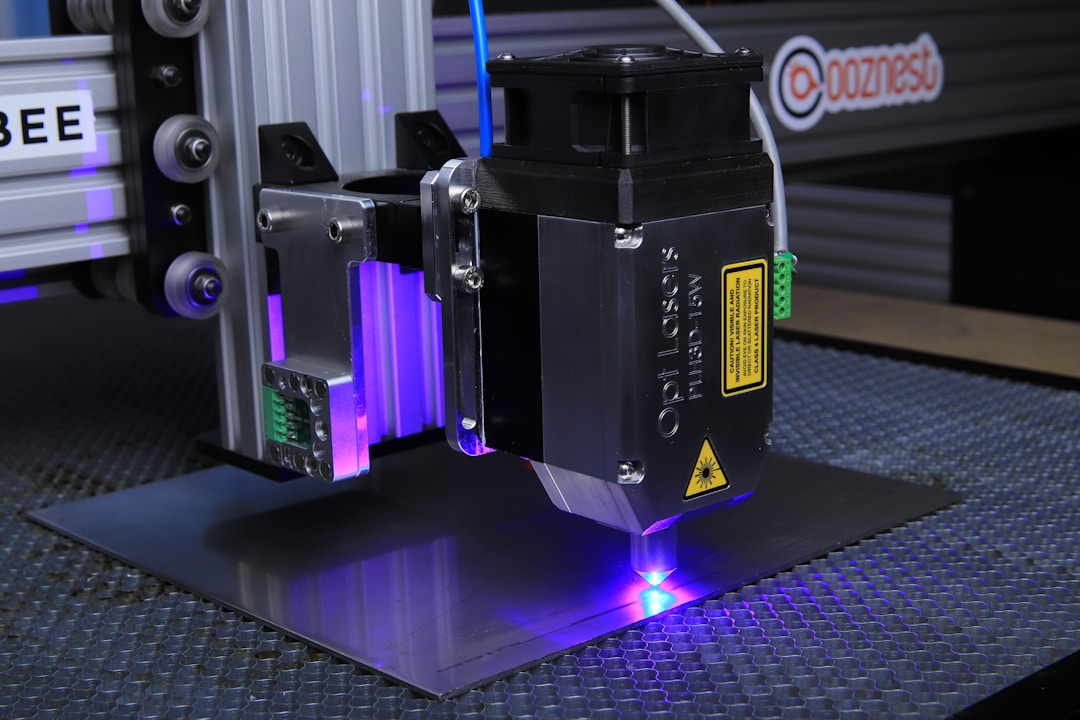
Femtosecond laser ablation causes the color of the stainless steel surface to change from silver gray to gray black (Figure 1a). The significant increase in light absorption means that under femtosecond laser treatment, rough surface microstructures may form on the sample surface. Figures 1b-f show stainless steel sheets subjected to femtosecond laser ablation (Λ=80) μ m) Surface scanning electron microscopy (SEM) images after. The bottom of the micro groove (Figure 1d) and the top surface of the micro peak (Figure 1f) are completely covered by periodic nanoscale ripples, which is a typical feature of laser irradiation and is known as laser-induced periodic surface structure (LIPSS). LIPSS is usually considered as the interference between the incident laser pulse and the scattered tangential wave generated by the front pulse.






 Language
Language